All cells use a number of different quality control mechanisms to ensure the functionality and stability of their respective protein diversity. Experts refer to this process as proteostasis. Proteostasis is made up of the two terms proteome (all the proteins that a cell can produce) and homeostasis (balance). Ideally, the proteins of a single cell are always correctly folded and always present in the right quantity. Despite all our noble efforts, our body does not manage to guarantee this optimum. In this article, we show you what ageing and some age-associated diseases have to do with a loss of proteostasis.
From DNA via the chain to the protein
First, we need to get a better understanding of the molecular structure of proteins. Every protein is made in a similar way. The first step, also known as transcription, takes place in the cell nucleus and involves reading and transcribing the blueprint from the DNA - our genetic material.
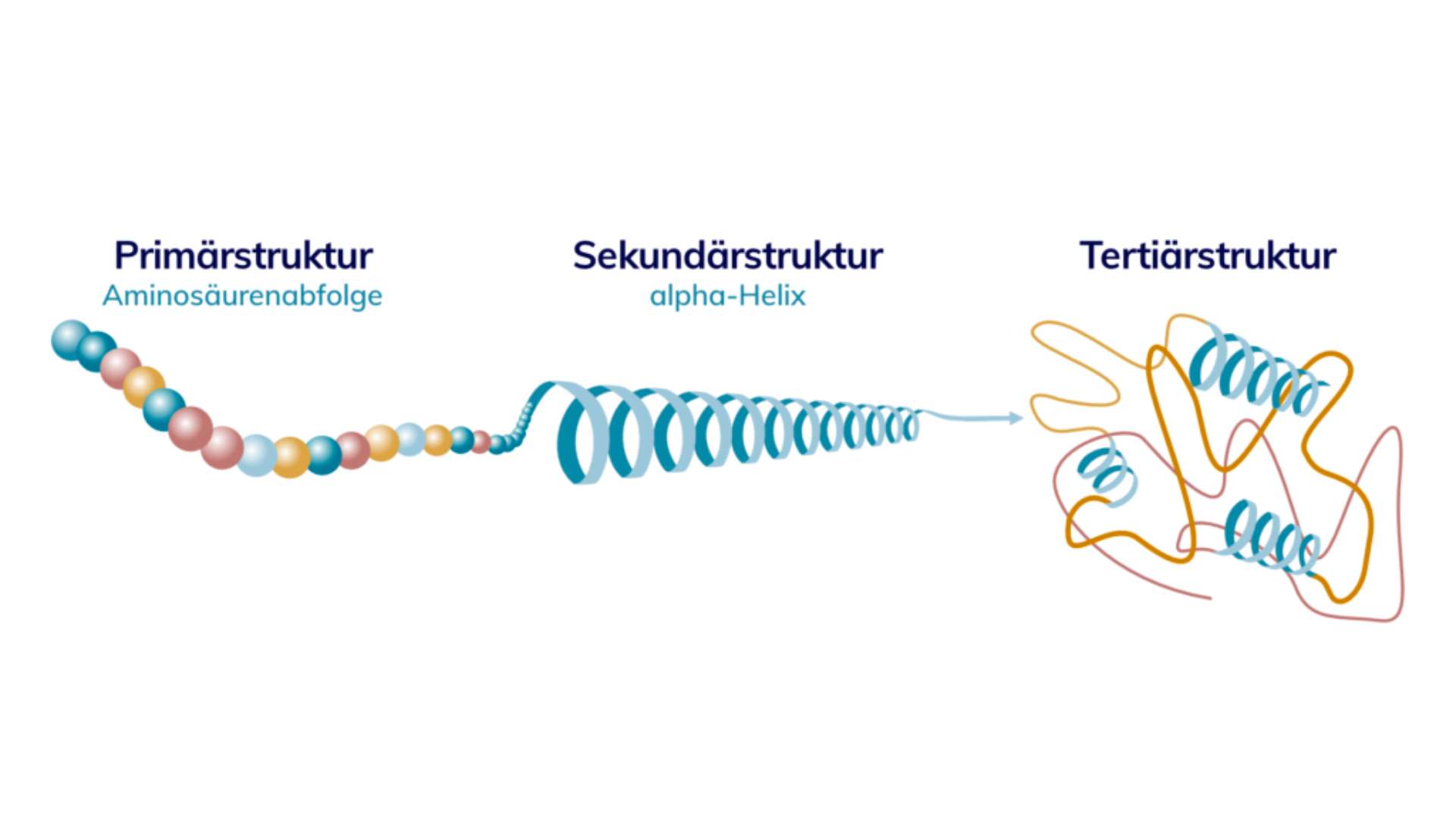
This transcribed information is then transported out of the cell nucleus andtranslated. This means that DNA language is translated into protein language, i.e. the protein is assembled on the basis of the DNA blueprint. A protein is then primarily a long linear chain of amino acids, similar to a string of pearls. This loose sequence of amino acids is called the primary structure.
In order for the proteins in their primary structure to start working, they still have to be folded, which is a very complex process. First, for example, the protein chain can be twisted to form a spiral, which is called an alpha-helix because of its typical shape. This form is the most common secondary structure. Through further folding steps, the proteins reach a three-dimensional form - the tertiary structure. In this state, they combine and work together with other proteins.
Did you know? The first amino acid to be discovered was cystine in 1810. It took until 1953 for an entire protein to be decoded in its amino acid sequence (primary structure). Frederic Sanger was able to decode the amino acid sequence of insulin.
Chaperones - the chaperones of our body
You can see from the diagram that the amino acid sequence as the primary structure is not enough. In order for the proteins in our body to perform their tasks, they require several intermediate steps. Only in the tertiary structure do the amino acids form a three-dimensional structure that is functional. A lot of work is needed before they get there.
New connections have to be made and sulphur-containing bridges are formed between individual amino acids. The whole process becomes very complex and is extremely error-prone. One wrong bond and the protein is unable to function. This is why there are several quality controls in our body to guarantee that everything is correct.
One of these quality controls are the chaperones. If someone is interested in England and language, the meaning is often already clear. A little tip: it is often used in the successful Netflix series "Bridgerton".
A chaperone is therefore an older woman who accompanies a younger one as a protector. A chaperone from England is then something like a chaperone protein in the body. It helps new proteins to fold or broken proteins to fold properly again.
Did you know? How many proteins are there in the world? The complexity of protein architecture is difficult to grasp. For this reason, scientists have developed an artificial intelligence that can predict the three-dimensional shape of a protein with a high degree of probability. "AlphaFold" was able to predict 215 million proteins and their tertiary structure in 2022 alone. The work of the researchers from Bael is considered to be one of the most important in recent years, as artificial intelligence can be used to develop drugs and vaccines more quickly in the future.
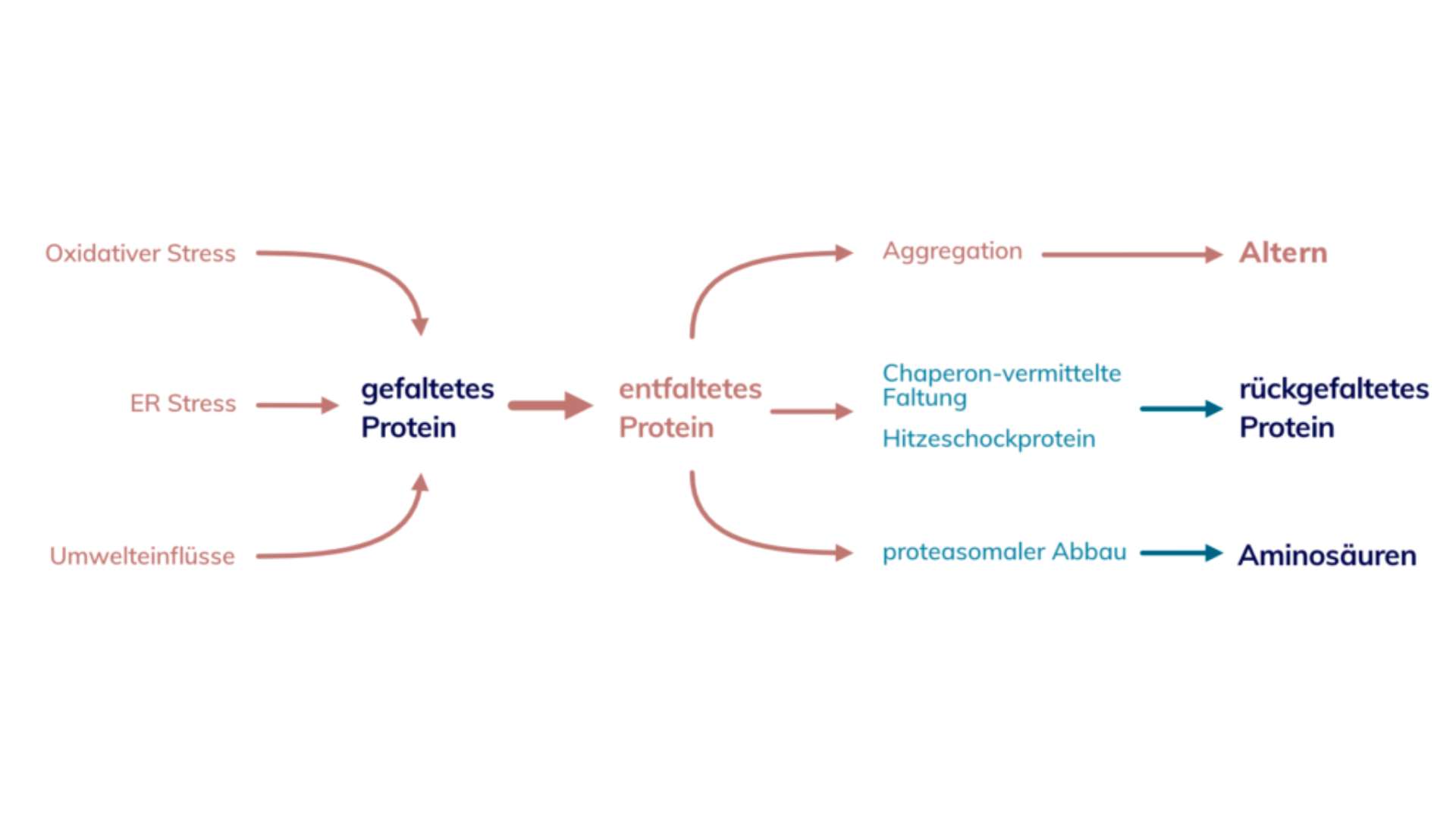
Loss of proteostasis - how do misfolded proteins occur?
Between 40 and 80% of all proteins are folded incorrectly and need help. That is an enormous number. There are several factors that can have a negative effect on the protein structure. These include ultraviolet radiation, heavy metals, heat or ethanol. It is therefore important to pay attention to the relevant certificates, especially when it comes to food or food supplements.
These environmental influences affect our proteins as well as our DNA. In addition oxidative stress - colloquially known as an excess of free radicals - affects the protein balance. But that's not all - there is also ER stress.
ER stands for endoplasmic reticulum, a device in each of our cells whose function could be described as a logistics center. This logistics center can become overloaded due to high demand and goods can no longer be delivered correctly. What would cause a lot of trouble for Amazon, Alibaba and co. is also dangerous for the cell. All the influences mentioned can cause proteins to unfold and thus become useless.
Proteostasis - how the body fights back
Proteostasis involves several mechanisms in an effort to maintain equilibrium. For the sake of clarity, we will focus on two of the most important mechanisms. In response to harmful environmental influences, the cell produces more proteins from the heat shock family. These are very resistant proteins that can stabilize other proteins in situations of cellular stress. They achieve this in interaction with chaperones.
If stabilization or restoration of correct folding is not successful, the proteins are useless for the time being and must be disposed of. What the waste incineration plant or recycling center does for us, the proteasome does in the body. Together with a small protein called ubiquitin (Ub), the broken molecule is marked several times, degraded and broken down into its individual amino acids.
All of these systems work in a coordinated manner to restore or dispose of misfolded proteins. This enables the body to prevent the accumulation of damaged components and ensure the continuous renewal of intracellular proteins. Autophagy is another component of our internal cellular waste disposal, which we will introduce to you in more detail as the 12th Hallmark of Aging.
So much for the theory. In practice, unfortunately, there is no guarantee that these sophisticated mechanisms will work at all times. The keyword time brings us to the next point.
Did you know? There are different heat shock proteins in our body. They are categorized according to their weight. As their name suggests, they are activated by heat, among other things. One of the best ways to do this is in infrared cabins or saunas. An increase in the concentration of heat shock proteins is associated with a number of health benefits.
In one study, the researchers were able to show that higher levels of Hsp70 were able to reduce the inflammatory mediator interleukin-10. This explains why sauna can help with inflammations such as arthritis.
Wrinkles - top for proteins, flop in old age
While we think of wrinkles as a sign of age and therefore rather negative, we now know that the opposite is true of proteins.
Many studies have shown that proteostasis changes with increasing age. The chronic accumulation of misfolded or unfolded proteins contributes to the development of some age-related diseases such as Alzheimer's disease, Parkinson's disease and cataracts. The frequency of these pathologies is constantly increasing due to rising life expectancy.
The production of chaperones in response to stress is also significantly reduced in old age. Studies on animal models support the hypothesis that chaperone depletion is the cause of a reduced lifespan. Genetically modified worms and flies, for example, which produce more chaperones, are particularly long-lived. An upregulation of some heat shock proteins was also found in long-lived mouse strains.
In addition, studies on mammalian cells show that upregulation of SIRT1 improves the heat shock response. SIRT1 belongs to the gene family of sirtuins, which are referred to as longevity pathways due to the numerous effects associated with ageing. Many other experiments and studies have provided scientific evidence for the link between chaperone quantity and lifespan, but it is beyond the scope of this article to list them all.
Proteostop
So medical-biological research has already shed a lot of light on proteostasis, but are there any tangible approaches to halting the age-related weakening of proteostasis? There are indeed many studies on this.
One approach aims to activate protein stability and folding mediated by chaperones. In the mouse model, drug induction of a specific heat shock protein preserved muscle function and slowed the progression of certain muscle diseases. In other model organisms, researchers have also used chaperones to improve age-related phenotypes. The chaperones of our body are therefore not only gentlewomen, but also fighters on the front line against ageing.
Another starting point is the proteasome and other mechanisms that serve to break down broken proteins, as studies show that the activity of these systems decreases with increasing age. This was achieved with selected enzymes that have developed their effect within this complex signaling pathway.
Supplementation with spermidine, for example, activated the autophagy system. This refers to the breakdown of damaged cell structures (such as proteins). In simplified terms, autophagy is similar in function to the proteasome we are familiar with.

Loss of proteostasis - outlook
Thus, there is ample evidence that ageing is associated with impaired proteostasis and that experimental disruption of proteostasis can induce age-related changes. In addition, there are promising examples of interventions that improve proteostasis and delay ageing in model organisms.
With the exception of cataracts, which can be cured by means of a minor surgical procedure, there are currently only inadequate treatment methods for pathologies such as Alzheimer's and Parkinson's disease. Both diseases are very stressful for those affected as well as their relatives and therefore also show how important it is to maintain research efforts in the field of proteostasis. Perhaps in the future it will be possible to broaden the horizon of proteostasis research from disease relevance to health relevance. After all, anything that can be prevented does not have to be treated later.
The next article in this series will focus on the fifth hallmark of ageing: deregulated nutrient measurement.
Literature
- López-Otín, Carlos et al. "Hallmarks of aging: An expanding universe." Cell vol. 186.2 (2023): 243-278. link
- Durairaj, Janani et al. "Uncovering new families and folds in the natural protein universe." Nature vol. 622,7983 (2023): 646-653. link
- Brunt, Vienna E, and Christopher T Minson. "Heat therapy: mechanistic underpinnings and applications to cardiovascular health." Journal of applied physiology (Bethesda, Md. : 1985) vol. 130,6 (2021): 1684-1704. link
- Pilch, Wanda et al. "The effects of a single and a series of Finnish sauna sessions on the immune response and HSP-70 levels in trained and untrained men." International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group vol. 40,1 (2023): 2179672. link
- Gressler, A Elisabeth et al. "Proteostasis in T cell aging." Seminars in immunology vol. 70 (2023): 101838. link
Graphics
The images were purchased under licence from Canva.